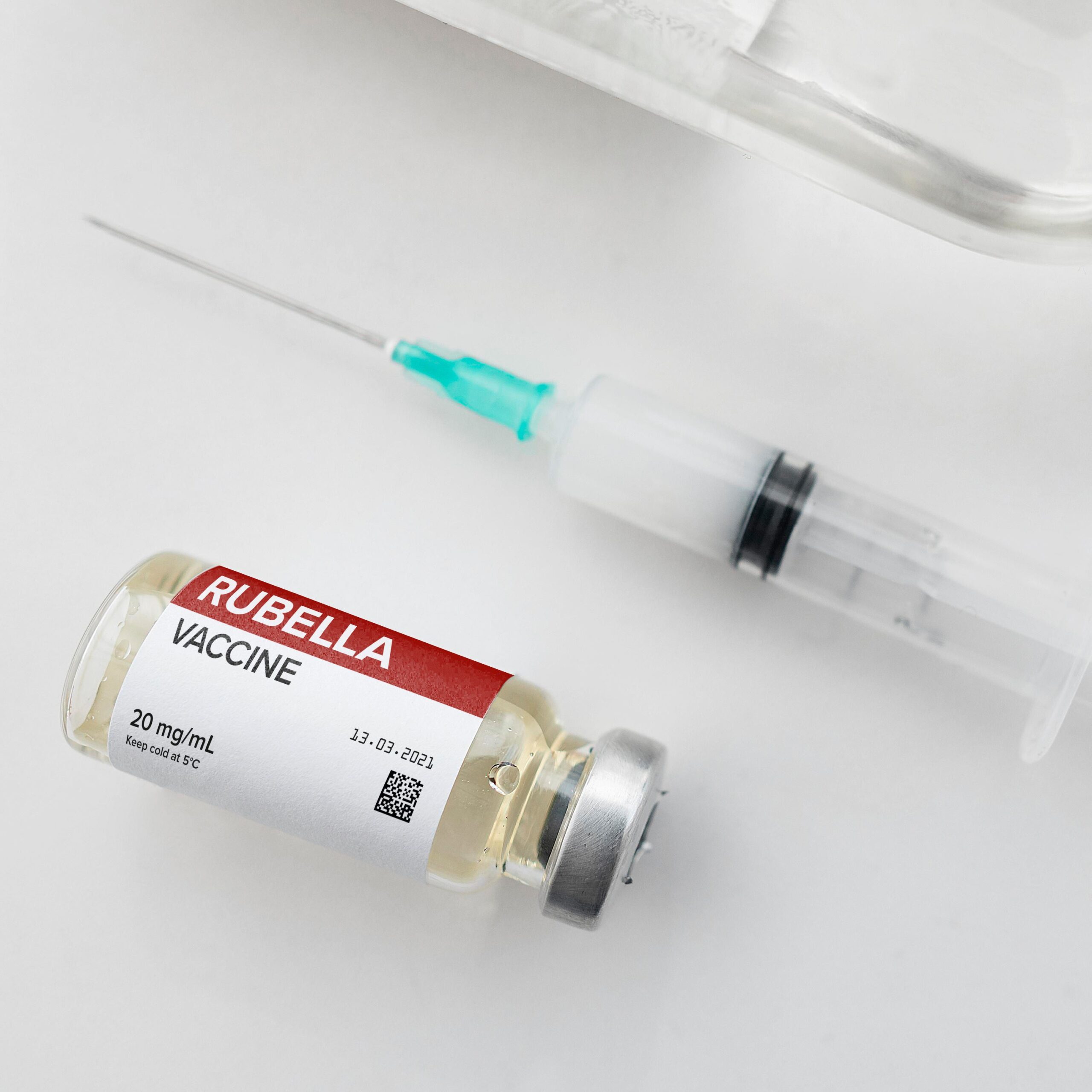
Patient safety is our priority
We know how important it is to ensure patient safety and, depending on the end use of the product, we follow different regulations. We comply with EU regulations and with the directive on counterfeit and falsified medicines (2011/62/EU) to ensure pharma label safety. Each product has a serial number and must be sealed at all times. We also use low migration materials to make sure our clients are never exposed to any danger.
Product security & label change management
This market requires certifications, rigid operating procedures and specialized materials that can withstand demanding conditions and assure security of supply, quality consistency and change management procedures. Besides, introducing or replacing an existing product is a process that can take years and has to go through product approval.
Product Security
Changes in the packaging of pharmaceutical products can pose a risk to patient health and safety. This is why we work to protect consumers, ensure supply-chain security and support compliance for the packaging of prescription drugs and high-risk over the counter medicines. Pharma product security consists in ensuring:
- Label security
- Tamper evident labels
- Anti-counterfeit labels
- Supply chain security
- Support compliance for packaging
- Smart security
Label Change Management
To let the validation process begin according to the rules we give our clients a minimum of 6 months advance notice. Every label should contain patient information, secure printability, product maintenance, security of information, a description of the process and temperature storage information.

Premiumization
The industry has to be functional, serving the needs of the patient in the security, labeling and externalization process. Our premium offer focuses on regular coated papers and special materials for hangers, tamper-evident and luminescent adhesives, which resist in extremely harsh conditions, avoiding migration of additives to the product.
A matter of size
Quality must be built into each stage of the manufacturing process, covering all aspects of production, from raw materials, facility and equipment to training and staff hygiene.
Long-lasting adhesion, high cohesion, content consistent with the information on the label, optical tracer to ensure no tampering has occurred: this is what our products offer, regardless of their size and shape.



